Recall Antigen Response as a Model for In Vitro T Cell Response
In vitro T cell-based assay is an important tool for functional characterization of immunomodulatory drug candidates. For example, in the field of immune-oncology (I/O), interest is focused on efforts to better understand and manipulate the immune system to either overcome tolerance or promote T cell killing of tumor targets. Conversely, agents that suppress T cell response are sought in inflammation/autoimmune research.
Several models of in vitro T cell response are often used in T cell-based assays. The list includes
- non antigen-specific T cell activation using anti-CD3/CD28 antibodies or other T cell activating agents such as phytohemagglutinin (PHA) and concanavalin-A (con-A).
- antigen-specific T cells generated by in vitro priming to activate and enrich them from peripheral blood T cell populations
- mixed-lymphocyte reaction (MLR), an allogeneic response to foreign major histocompatibility complex protein.
- In vitro recall antigen response by memory T cells induced in vivo from previous exposure through virus infection (e.g., cytomegalovirus [CMV]), or vaccination (e.g., tetanus toxoid)
The current article discusses the use of recall antigen response assay as a model for in vitro T cell response.
In Vitro Recall Antigen Response
Following clearance of an invading pathogen e.g., viruses or bacteria, as well as after a series of vaccinations against pathogenic antigens, memory T cells and B cells are established in both lymphoid and peripheral sites. When a secondary infection occurs, a robust recall T cell and humoral response to the antigens can be elicited, resulting in a faster and more efficient elimination of the infecting pathogen.
This strong response is also shown in vitro in a recall antigen assay. For example, within 4-7 days of the start of the assay, the memory T cell response to a recall antigen can be detected and analyzed (e.g., interferon-gamma secretion, cell proliferation etc.). Measuring a recall T cell response in the presence of a test compound is a useful method for determining the immunomodulatory potential of the compound. Examples of recall antigens frequently used in vitro assays include human cytomegalovirus (HCMV), a member of the herpesvirus family with a high seroprevalence in the population, and tetanus toxoid, against which most people are routinely vaccinated.
Peripheral blood mononuclear cells (PBMC) is the most common cell population used in a recall antigen assay, as it provides both effectors (T cells) as well as stimulators or antigen-presenting cells (e.g., monocytes, B cells). For a successful recall antigen assay, it is important to select a well-characterized donor and PBMC, particularly in regard to the presence of memory T cells to the recall antigen of interest. For example, all of Ignyte Bio’s donors have been screened for previous HCMV exposure. In addition, all of Ignyte Bio’s PBMC products have been tested for T cell reactivity to the recall antigens HCMV and tetanus toxoid.
Figure 1 below shows a summary of HCMV and tetanus toxoid recall response from several different Ignyte Bio donors. Both assays are routinely performed for each PBMC lot, and the results are published in each certificate of analysis (COA). Figure 1a shows different levels of IFN-gamma secretion after incubation with HCMV antigen. Negative IFN-gamma recall response from donors 103 and 108 are consistent with their HCMV seronegative status. Different levels of IFN-gamma response are shown among the different HCMV seropositive donors. Figure 1b shows recall responses to tetanus toxoid from these donors. Availability of recall data from each PBMC lot allows customers to evaluate and choose the suitable donor PBMC for their assays.
In addition to PBMC, recall antigen assays can also be conducted using autologous effector/stimulator cell pairs. Ignyte Bio can help you source the appropriate effector cell populations such as: pan CD3+ T cells, CD4+ T cells or CD8+ T cells, and stimulator populations such as irradiated PBMC, monocytes, or dendritic cells (DC) from the same donor.
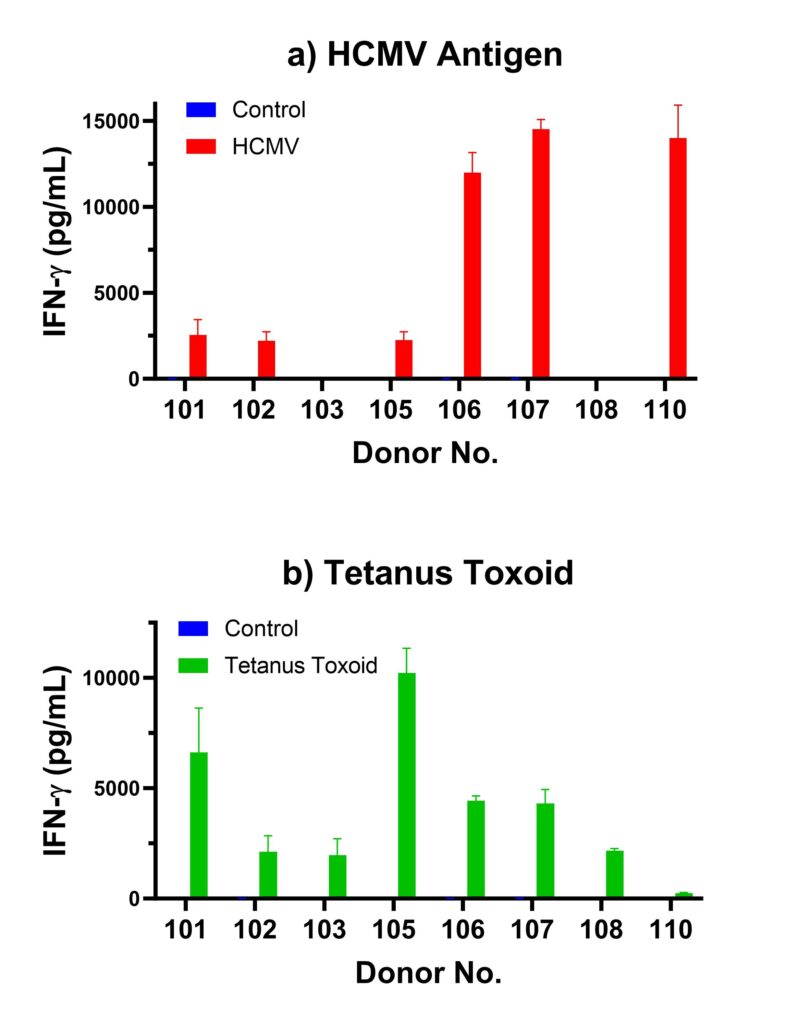
Figure 1. Recall Response of Different Ignyte Bio Donors to (a) HCMV Antigen and (b) Tetanus Toxoid. Cryopreserved PBMC were thawed and plated in the presence or absence of 1 ug/mL HCMV Antigen or 1 ug/mL Tetanus Toxoid in a round bottom 96-well plate. Each culture condition was done in triplicates. After 4 days of incubation in a 37 C, 5% CO2 incubator, culture supernatant was harvested and analyzed for IFN-gamma using the IFN-gamma LUMIT kit (Promega, Madison, WI).
