Anti-HPV E7 T Cells and the Fight Against Cervical Cancer
Cervical cancer induced by human papillomavirus (HPV) remains a global concern with high mortality rates, especially for women in under-developed and under-served portions of the world.
According to the CDC, over 47,000 HPV-associated cancers occur in the United States each year, with women representing about 55% of those cases.
Globally, cervical cancer impacts are felt more heavily in low- and middle-income countries. According to the World Health Organization:
- There were an estimated 604,000 new cervical cancer cases and 342,000 deaths in 2020, the 4th most common cancer among women
- About 90% of cervical cancer cases occur in low- and middle-income countries with limited access to screening, vaccination, and treatment
Thus, researchers continue to look for low-cost, effective preventative approaches and therapeutics to protect against and treat HPV infections and cervical cancer.
Exploring the Potential of Oncogenic Proteins E6 and E7
Oncogenes E6 and E7 have been shown to play a pivotal role in the establishment and progression of cervical cancer, and thus are ideal targets for vaccine and therapy research.
Together, the E6 and E7 oncoproteins can induce the main hallmarks of cancer:
- Sustaining proliferation
- Evading growth suppressors
- Activating invasion and metastasis
- Enabling replicative immortality
- Inducing angiogenesis
- Resisting cell death
HPV E7 was the first HPV oncogene to be discovered. As described in Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy:
“It is a relatively small phosphoprotein of about 100 amino acids, with three conserved regions 1/2/3 (CR1/2/3). A small portion of CR1 and nearly entire CR2 from the amino terminal holds sequence similarity with adenovirus (Ad) E1A proteins and large T antigen of SV40 (Phelps et al., 1988). The CR2 domain is composed of poorly conserved sequence followed by the CR3 region. The CR3 region at the carboxyl terminal end is conserved and encodes a zinc finger domain containing two CXXC motifs separated by 29 amino acid residues (Barbosa et al., 1990; McIntyre et al., 1993). It is responsible for the zinc-dependent dimerization and for mediating E7 interaction with cellular proteins responsible for cell cycle regulation and apoptosis (p21 and pRb; Ohlenschlager et al., 2006).”
Anti-HPV E7 T Cells for Cervical Cancer Research
Both E6 and E7 have been linked to several pathways that influence tumorigenesis and are our most promising and effective targets for cervical cancer therapeutics. Current clinical research focusing on E6 and E7 includes vaccines, genome editing technologies, immunotherapies, and phytotherapies.
One of the E7 peptides, HPV E711-20 (YMLDLQPETT), has been identified as one of the epitopes for CD8+ T cells detected in individuals with cervical cancer. We have developed CD8+ T cells specific for HPV E711-20 (YMLDLQPETT) in association with HLA-A*0201.
Antigen specificity of our anti-HPV E7 T cells is demonstrated by tetramer binding as well as functional studies (specific IFN-γ release and cytotoxicity).
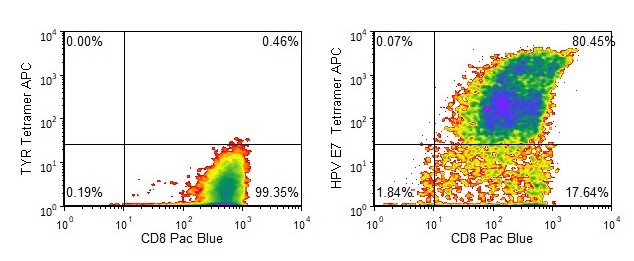
Figure 1. Tetramer Analysis. Cryopreserved T cells were thawed and stained with APC-labelled HLA-A-02:01-HPV E711-20 (YMLDLQPETT, HPV E7 Tetramer APC) or a control tetramer HLA-A-02:01-tyrosinase369-377 (YMDGTMSQV, TYR Tetramer APC). These cells were counterstained with anti-CD8 Pacific Blue reagent and analyzed by flow cytometry. Non-viable cells were excluded from the analysis using 7-AAD.
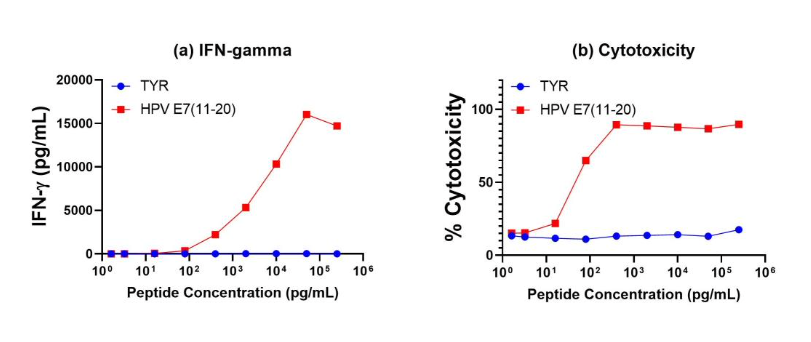
Figure 2. Antigen-Specific IFN-γ Secretion and Cytotoxicity Data. Freshly thawed anti-HPV E7(11-20) CD8+ T cells were plated at 2 x 104 cells/well in the presence of 2 x 104 T2 cells, an HLA-A-02:01+ B-LCL (ATCC, Manassas, VA) and different concentrations of HPV E711-20 (YMLDLQPETT) or tyrosinase369-377 (YMDGTMSQV) control (TYR) peptide in a 96-well round bottom plate. After 18-24 hours of incubation at 37°C, 5% CO2, culture supernatants were harvested and assayed for IFN-γ using the Lumit™ IFN-γ Immunoassay (Promega, Madison, WI). IFN-γ concentration is plotted against HPV E711-20 and control peptide concentrations (figure 2a). Remaining cells in each well were stained with the viability dye 7-AAD and analyzed using flow cytometry. % Cytotoxicity (=% 7-AAD+ target cells) is plotted against HPV E711-20 and control peptide concentrations (figure 2b).
