Antigen-Specific T Cells to MUC1 Antigen
Our scientists were among the first to develop custom antigen-specific T cells for research use and have created them against a diverse range of targets, including viral, tumor, and self-antigens.
We’re now developing the next generation of antigen-specific T cells to target some of today’s most prevalent and challenging diseases. More on that below. But first, a primer on antigen-specific T cells.
Skip the Primer – Show Me What’s New!
The Role of Antigen-Specific T Cells in Disease Research and Treatment
T cells are a major player in the immune response in various diseases, including cancer and pathogenic infections, and are mediators in inflammation and autoimmunity. A large body of research has been conducted over the past few decades to manipulate T cell responses, leading to many clinical strategies including cancer vaccines, T cell adoptive therapy, CAR-T therapy, immune-oncology, and the use of regulatory T cells for autoimmune diseases.
Antigen-specific T cells are an important tool in T cell biology research and potency assays. Investigators can isolate them directly from the disease site (e.g., tumor-infiltrating lymphocytes (TIL) or inflammatory lesion infiltrate populations), but these procedures often yield a limited number of cells due to low cell frequency and lack of robust isolation methods.
Alternatively, antigen-specific T cells can be generated in vitro using antigen-presenting cells pulsed with whole antigens or peptides. However, this process is often time-consuming with a low success rate.
About Ignyte Bio’s Antigen-Specific T Cells
Ignyte Bio offers antigen-specific T cells for research use you can rely on.
- Typically generated using multiple in vitro stimulations with peptide antigens
- Not immortalized or genetically modified, so they more closely mimic physiological T cells
- Specificity analyzed using the cognate peptide/MHC tetramer binding as well as interferon-gamma (IFN-γ) secretion assays.
Announcing Ignyte Bio’s Newest Antigen-Specific T Cell : anti-MUC-1 CD8+ T Cells
MUC1 (Mucin1), also known as EMA, MCD, PEM, PUM, KL-6, and MAM6, is the best characterized transmembrane mucins. It has a variable number of highly glycosylated, 20-amino acid tandem repeats (VNTR), a sperm protein-enterokinase-agarin (SEA) domain (extracellular), a transmembrane domain, and a 72-amino acid cytoplasmic tail domain that extends up to 200–500 nm out of the cell surface. It is overexpressed and differentially glycosylated in various epithelial adenocarcinomas such as lung, liver, colon, breast, pancreatic, and ovarian cancer, and also hematological malignancies such as acute myelogenous leukemia (AML) and multiple myeloma (Brossart et. al., 2001). MUC1-based cancer immunotherapy approaches evaluated to date include antibody- and vaccine-based therapies (Chen et. al., 2021).
Using MHC-binding motif algorithms in combination with in vitro T cell stimulation strategies, as well in vivo vaccination of transgenic mice, several cytotoxic T-lymphocyte (CTL) epitopes were identified. CTLs specific to a few of these peptides, presented by HLA-A*0201, have been shown to recognize and lyse breast cancer cells expressing MUC1 (Carmon et. al., 2000 and Brossart et. al., 1999)
Ignyte Bio’s anti-MUC1-specific CD8+ T cells were generated against the peptide MUC113-21, (LLLTVLTVV).
Figure 1 shows specific recognition of the MUC113-21 in association with HLA-A-02:01 as shown by the tetramer binding study. Figure 2 shows specific IFN-γ secretion and specific lysis when HLA-A-02:01+ target cells were incubated with the MUC113-21 peptide but not the control MART-126-35, 27L peptide.
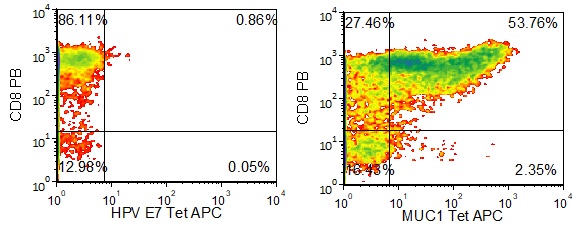
Figure 1. Recognition of Antigenic Peptide MUC113-21 Demonstrated by Peptide/MHC Tetramer Binding Analysis. Cryopreserved T cells were thawed and stained with APC-labelled HLA-A-02:01-MUC113-21(LLLTVLTVV, MUC1 Tetramer APC) or a control tetramer HLA-A-02:01-HPV E711-20(YMLDLQPETT, HPV E7 Tetramer APC). These cells were counterstained with anti-CD8 Pacific Blue reagent and analyzed by flow cytometry. Non-viable cells were excluded from the analysis using 7-AAD.
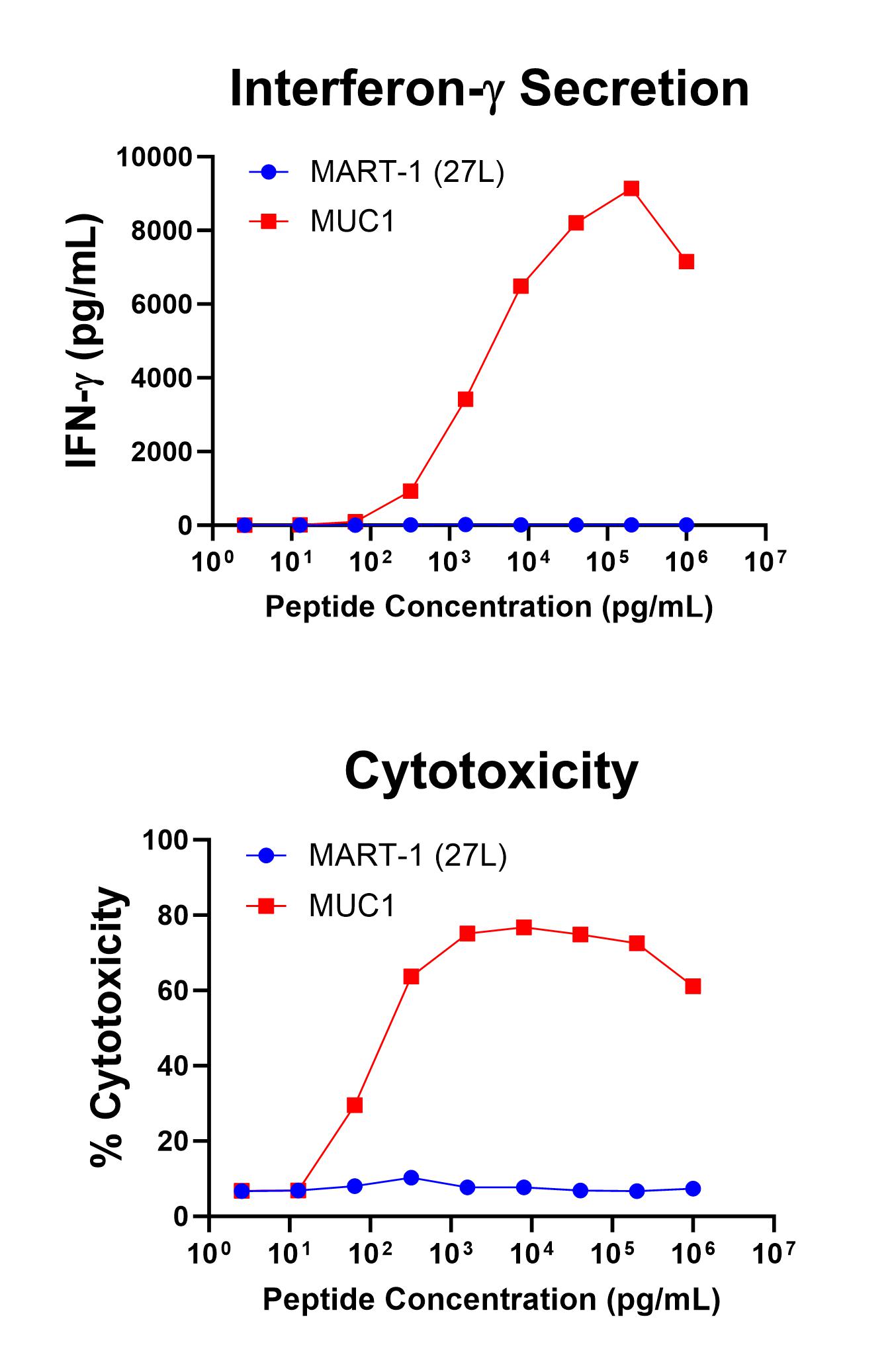
Figure 2. Antigen-Specific IFN-γ Secretion and Cytotoxicity Data. Freshly thawed anti-MUC1 T cells were plated at 2 x 104 cells/well in the presence of 2 x 104 T2 cells, an HLA-A-02:01+ B-LCL (ATCC, Manassas, VA) and different concentrations of MUC1 13-21 (LLLTVLTVV): MUC1, or MART-126-35, 27L (ELAGIGILTV): MART-1 (27L) control peptide in a 96-well round bottom plate. After 18-24 hours of incubation at 37oC, 5% CO2, culture supernatants were harvested and assayed for IFN-g using the Lumit™ IFN-γ Immunoassay (Promega, Madison, WI). IFN-γ concentration is plotted against MUC1 and control peptide concentrations (upper graph). Remaining cells in each well were stained with the viability dye 7-AAD and analyzed using flow cytometry. % Cytotoxicity (= % 7-AAD+ target cells) is plotted against the respective peptide concentrations (lower graph).
