FAQs on Using Dendritic Cells for Immuno-oncology and Autoimmunity Research and Development
Dendritic cells are a family of immune cells that help to maintain balance in the immune system. The dendritic cell family is typically divided into four subgroups:
- Conventional dendritic cells (DC1 and DC2)
- Plasmacytoid dendritic cells
- Inflammatory dendritic cells
- Langerhans cells
Dendritic cells serve two main functions:
- Antigen-presenting cells: As efficient antigen-presenting cells, dendritic cells activate immune effectors, such as T cells and B cells in response to pathogens, tumors, mismatched tissue transplants, and other foreign bodies.
- Self-regulators: Dendritic cells have the unique ability to make the immune system tolerant or unresponsive to self-antigens, helping to prevent over-active immune responses or call off immune responses once no longer needed — returning the immune system to a steady-state.
Without the proper function of dendritic cells as antigen-presenters, there can be uncontrollable infection or tumor growth. And without their self-regulating capabilities, widespread inflammation or autoimmune disease can present.
In humans, dendritic cells represent only 0.1%-0.5% of mononuclear cells in peripheral blood. While this low abundance previously limited studies with human dendritic cells, the method for generating dendritic cells in vitro from more abundant precursors, such as monocytes, changed that. Now, monocyte-derived dendritic cells are commercially available in large numbers as a valuable tool in both immuno-oncology and inflammation research.
Ignyte Bio is lucky to have a resident dendritic cell expert! Benjamin Tjoa, Ph.D., Chief Scientific Officer, holds two dendritic cell-related US patents and has been published in numerous journals.
Below, Ben answers some common questions about dendritic cells.
Dendritic Cell FAQs
- How do dendritic cells present antigens and activate T cells?
“Dendritic cells are the most efficient antigen-presenting cells. They take up antigens and pathogens, generate MHC-peptide complexes, migrate from the sites of antigen acquisition to secondary lymphoid organs and, finally, they physically interact with and stimulate T lymphocytes.”
Read more about The cell biology of antigen presentation in dendritic cells by Thery and Amigorena.
- How do dendritic cells recognize foreign antigens?
Signals from the surrounding cells or microenvironment play a major role in determining the nature of the immune response driven by dendritic cells. For example, when dendritic cells encounter pathogens or other signals associated with “danger” such as inflammatory cytokines or products of damaged tissues, they undergo an activation process at which point they acquire the capability to activate immune response.
- How do dendritic cells enter lymph nodes?
Lymphatic vessels transport peripheral fluids, including innate immune cells, into the lymph nodes where they can initiate an immune response to fight pathogens.
Learn more about the two routes immune cells can take into lymph nodes, including high endothelial venules and afferent lymphatic vessels, in Lymphatic Migration of Immune Cells by Hampton and Chtanova.
- When do dendritic cells inhibit an immune response?
Unlike the highly efficient T cell activation process, dendritic cells can become poor T cell activators in the presence of anti-inflammatory factors such as transforming growth factorb-(TGF-β), IL-10, corticosteroids, or other factors. In some cases, dendritic cells can induce regulatory T cells that prevent the proliferation of effector T cells.
- How do dendritic cells phagocytose?
“Dendritic cells are outstanding antigen presenting cells due to their robust ability to internalize extracellular antigens using endocytic processes such as receptor-mediated endocytosis, phagocytosis, and macropinocytosis.”
Read more about these processes in Macropinocytosis in phagocytes: regulation of MHC class-II-restricted antigen presentation in dendritic cells by Liu and Roche.
Dendritic Cells for Immuno-oncology and Autoimmunity Research
The sensitivity of dendritic cells has made them a popular research emphasis in both the immuno-oncology and inflammation/autoimmunity fields. The tumor microenvironment contains many factors that affect dendritic cell function, including those that affect dendritic cell differentiation from CD34+ progenitor cells such as tumor-derived vascular endothelial growth factor (VEGF) and gangliosides, as well as factors that suppress dendritic cell activation such as IL-6, IL10, prostaglandin E2 (PGE-2), and TGF-β. Efforts to block or reverse these suppressive effects are hot topics in immuno-oncology research.
Dendritic cells are also at the center of inflammation/autoimmunity research for their role in the generation of inflammatory Th17 cells.
Now Available: Monocyte-Derived Dendritic Cells
Ignyte Bio offers immature dendritic cells differentiated in vitro from peripheral blood monocytes.
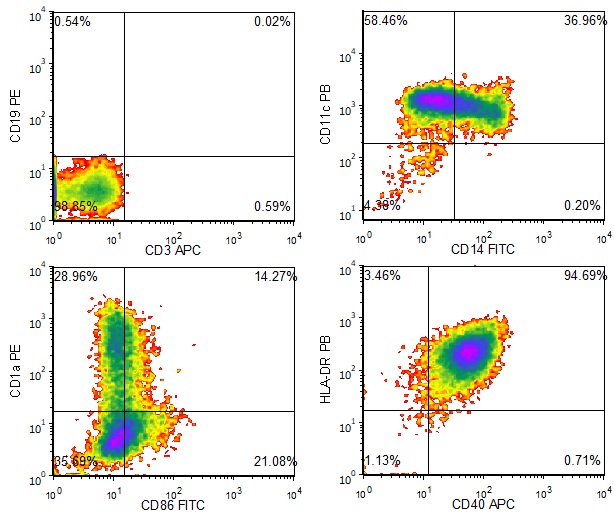
Figure 1. Phenotype of Immature Dendritic Cells. Ignyte Bio’s immature dendritic cell products are characterized by immunophenotyping. This cell type is CD3-, CD19-, CD11c+, CD14-/low, CD86low, HLA-DR+, and CD40+/low. Some of the cells in this population express CD1a.
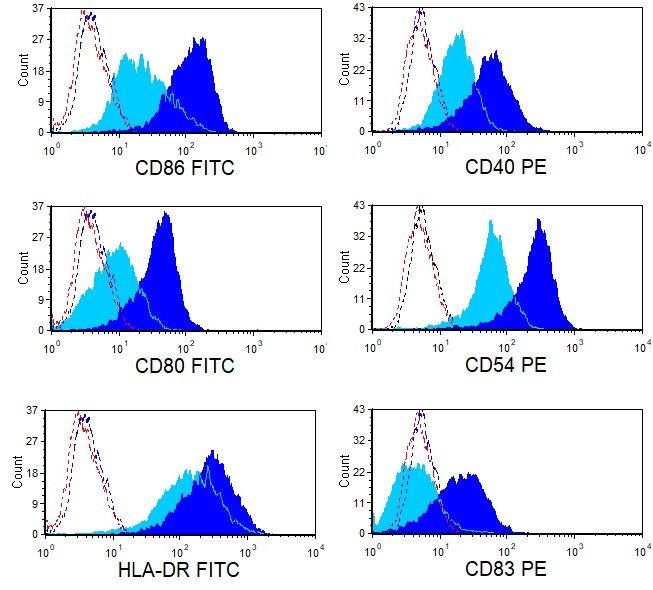
Figure 2. Phenotype of Mature Dendritic Cells. Ignyte Bio’s immature dendritic cells can be induced to mature in vitro by incubating them in the presence of various dendritic cell maturation factors. This figure shows differential expression of several cell surface proteins on immature dendritic cells (light blue) vs mature dendritic cells (dark blue) as analyzed using flow cytometry. Dotted curves represent background staining.
