Donor-to-Donor Variation in Response to 3 Critical Recall Antigens
Peripheral blood mononuclear cells (PBMC) are useful tools for evaluating immune response because they represent most of the resident immune cells in a donor’s sample, including T cells, B cells, natural killer (NK) cells, and monocytes.
Varying Responses to Today’s Most Relevant Antigens
In addition to cell count, viability, and flow cytometric data showing percentages of each component of the population, each of our PBMC lots are tested for immune response to a panel of antigens commonly found in blood donors and relevant to modern research needs:
- Tetanus Toxoid
- Human Cytomegalovirus (HCMV)
- SARS-CoV2
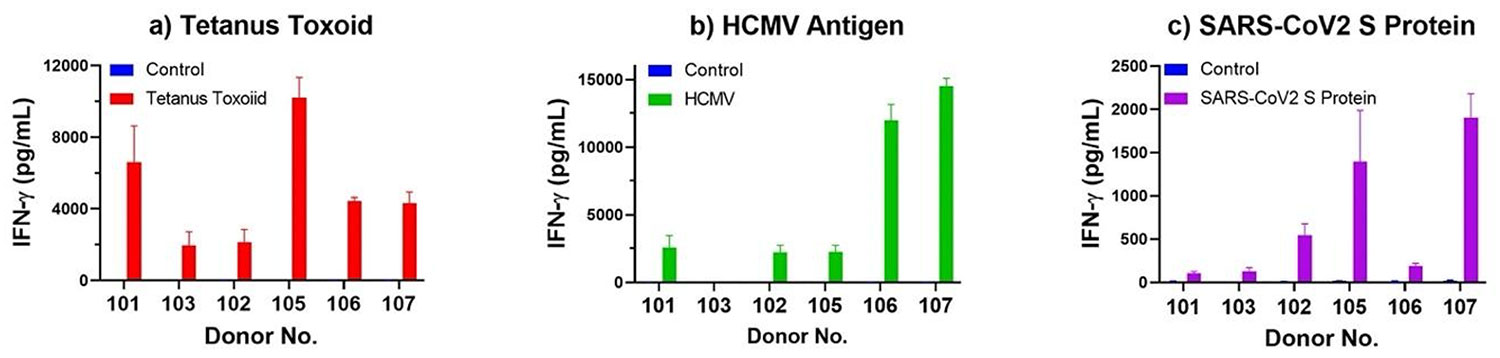
The below analysis of select Ignyte Bio donors highlights the donor-to-donor variation in response to these three critical recall antigens. The results of an in vitro antigen-specific response (IVAR) assay for these three recall antigens is included in the Certificate of Analysis with every Ignyte Bio PBMC product.
Read more about the detailed contents of our Certificates of Analysis here.
Figure 1. In Vitro Antigen-Specific Response (IVAR) Assay Demonstrates Varying Donor Responses to Recall Antigens. Equal number of PBMC from different donors were plated in 200 μL X-VIVO15 medium (Lonza, Gaithersburg, MD) in the presence or absence of antigen (tetanus toxoid, cat. no. AG-02, HCMV antigen , cat. no. AG-01, or SARS-CoV-2 (S) peptide pool. After 4 days of incubation in a 37°C, 5% CO2 Incubator, culture supernatants were assayed for IFN-γ using the Lumit IFN-γ Immunoassay (Promega, Madison, WI). IFN-γ level from select donors’ response to each antigen are presented on the bar graphs above.
Interested in additional recall antigen testing? We can test our donors for response to a variety of antigens or source a donor that fits your exact specifications.