MLR (Mixed Lymphocyte Reaction) As A Model for In Vitro T Cell Response
In vitro T cell-based assay is an important tool for functional characterization of immunomodulatory drug candidates. For example, in the field of immune-oncology (I/O), interest is focused on efforts to better understand and manipulate the immune system to either overcome tolerance or promote T cell killing of tumor targets. Conversely, agents that suppress T cell response are sought in inflammation/autoimmune research.
Several models of in vitro T cell response are often used in T cell-based assays. The list includes
- non antigen-specific T cell activation using anti-CD3/CD28 antibodies or other T cell activating agents such as phytohemagglutinin (PHA) and concanavalin-A (con-A).
- in vitro memory T cell response to recall antigens generated in vivo from previous exposure through virus infection (e.g., cytomegalovirus [CMV]), or vaccination (e.g., tetanus toxoid)
- antigen-specific T cells generated by in vitro priming to activate and enrich them from peripheral blood T cell populations
- mixed-lymphocyte reaction (MLR), an allogeneic response to foreign major histocompatibility complex protein.
The current article discusses the use of MLR as a model for in vitro T cell response.
Mixed-Lymphocyte Reaction (MLR)
The principle of a mixed-lymphocyte reaction (MLR) is the ability of T cells from one individual to recognize a mismatch of the major histocompatibility complex (MHC) from cells from a different individual (alloantigen). This type of response, also known as an allogeneic response, is very rigorous as shown in the rejection of transplanted organs from a donor with mismatched MHC.
This strong response is also shown in vitro in an MLR. Within 5-7 days of the start of the culture, the allogeneic T cell response in MLR assay can be analyzed (e.g., interferon-gamma secretion, cell proliferation etc.). Measuring MLR in the presence of a test compound is a useful method for determining the immunomodulatory potential of the compound.
An MLR assay can be designed using a variety of effector and stimulator pairs. Effector populations can be PBMC, pan CD3+ T cells, CD4+ T cells or CD8+ T cells. Stimulator populations can be mitomycin-C treated PBMC, irradiated PBMC, monocytes, or dendritic cells (DC). It is important to compare donors’ MHC (HLA) haplotypes to assure the presence of sufficient mismatches between the effector and stimulator populations.
Below we present an MLR experiment which compares allogeneic T cell responses (donor A) when co-cultured with 3 different stimulator populations from the same donor (donor B): monocytes, immature DC and mature DC (Figure 1). This data clearly shows the superior capacity of mature DC, compared to immature DC and monocytes, in stimulating allogeneic T cell response.
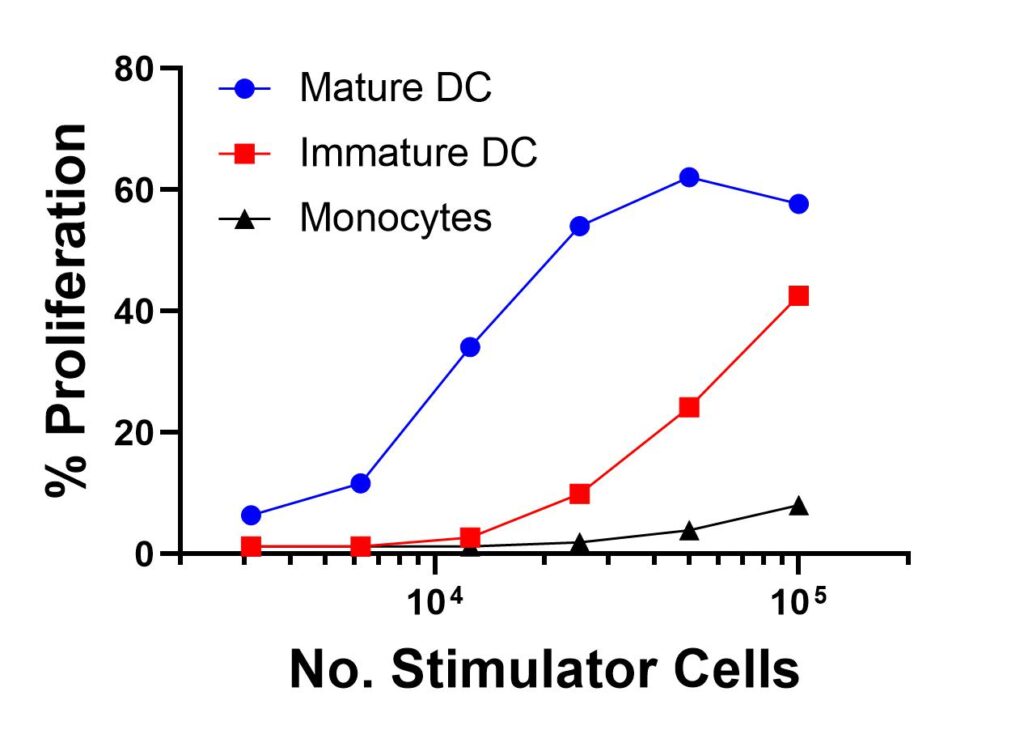
Figure 1. Comparison of Three Different Stimulator Cell Types in an MLR. A total of 4 x 105 CFSE-labelled CD3+ purified T cells from donor A were incubated in the presence or absence of 105, 5 x 104, 2.5 x 104, 1.25 x 104, 6.3 x 103 or 3 x 103 APC (monocytes, immature dendritic cells (DC) or mature DC) from an allogeneic donor B for 5 days in a 37 deg C/5% CO2 incubator. Mature DC was generated by culturing cryopreserved immature DC in the presence of 20 ug/mL poly IC for 48 hours prior to use in MLR. At the conclusion of the incubation period, each population was harvested, labelled with phycoerythrin (PE) –labelled anti-CD4 antibody, and analyzed on a flow cytometer. For the current assay, only CD4+ population was analyzed.
